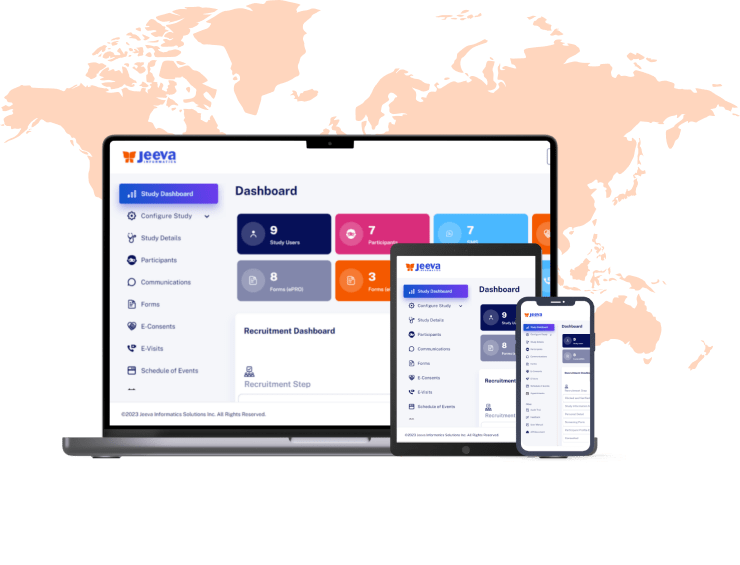
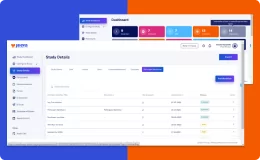
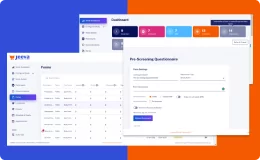
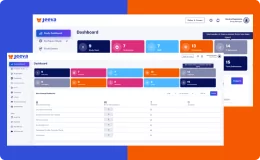
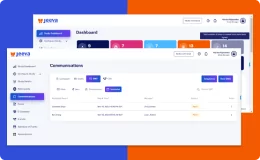
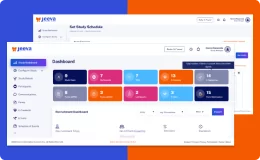
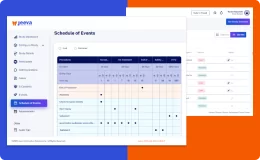
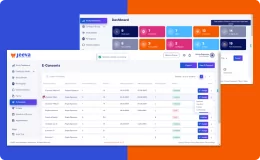
Jeeva Clinical Trials stands out with its unified, cloud-based platform designed for rapid study configuration, seamless mid-study amendments, and robust patient engagement tools. Our AI-powered insights, integrated eConsent, user-friendly interfaces, centralized data management, and compliance with global regulations enable faster, more efficient, and patient-centric clinical trials. The platform supports remote and decentralized trials, making it a future-ready solution for sponsors, CROs, and research sites.