
CAR-T To N-of-1 Studies: How Jeeva Is Helping Drive Trial Efficiencies
Source: SCRIP CITELINE COMMERCIAL Jeeva’s founder and CEO talks to Scrip about how the firm’s clinical trial management platform does away with the need for
Jeeva Trials > News

Source: SCRIP CITELINE COMMERCIAL Jeeva’s founder and CEO talks to Scrip about how the firm’s clinical trial management platform does away with the need for
Source: PRWeb Remarkable progress in the development of cell and gene therapies (CGTx) has ignited hope for millions of patients battling debilitating human diseases including Central

Source: PM360online Enhancing patient engagement in rare disease support programs requires tailored strategies that leverage innovative approaches, including AI and decentralized trials: Personalized Communication: Utilize
Source: IG Living Magazine Research and development of orphan drugs is time-consuming and costly, and appropriate participants for clinical trials are hard to find. Efficiently
Source: PR Web President Biden’s loss of his beloved son to brain cancer in 2015 inspired the Cancer Moonshot initiative. Dr. Harsha Rajasimha’s inspiration for
Source: PRWeb Jeeva Clinical Trials is revolutionizing drug development by integrating AI and automated workflows into clinical trials, significantly reducing the time and cost of

Source: Western Pennsylvania Healthcare News The recent Food and Drug Administration (FDA) approval of cell-based gene therapies for Sickle Cell Disease (SCD) marks a groundbreaking

Source: Rare Disease and Orphan Drug Journal Abstract One of the main challenges in rare diseases is the unavailability of reliable estimates of prevalence and
Source: Dermatology Times Gender gaps, with consistent underrepresentation of females, present a substantial challenge in clinical research.(1) Achieving a balanced representation of both genders has

Source: The Company of Biologists Rare diseases affect only a few people in a given population but, collectively, they impact over 400 million people globally

Source: Polite On Society Greetings, everyone. Diversity in medicine in today’s society is important. This week, we hear from Dr. Harsha Rajasimha, Ph.D., Founder and

Source: PRWeb The Food and Drug Administration has approved 16 new gene therapies to date, but for most people around the world, these expensive treatments

Source: PRWeb The cost of patients dropping out of clinical trials continues to be a major problem for the development of new drugs and treatments

Source: PRWeb Dr. Harsha Rajasimha, Founder and CEO of Jeeva Trials, highlights the importance and value of Investigator-Initiated Trials (IITs) in gathering real-world data on

Source: Onco’Zine The Inflation Reduction Act of 2022 (IRA) covers new and reinstated tax laws that will affect individuals and businesses. Through IRA, President Joe
Source: PRWeb Drug pricing controls imposed by the Inflation Reduction Act of 2022 may appear positive for patients in the short term. However, these controls
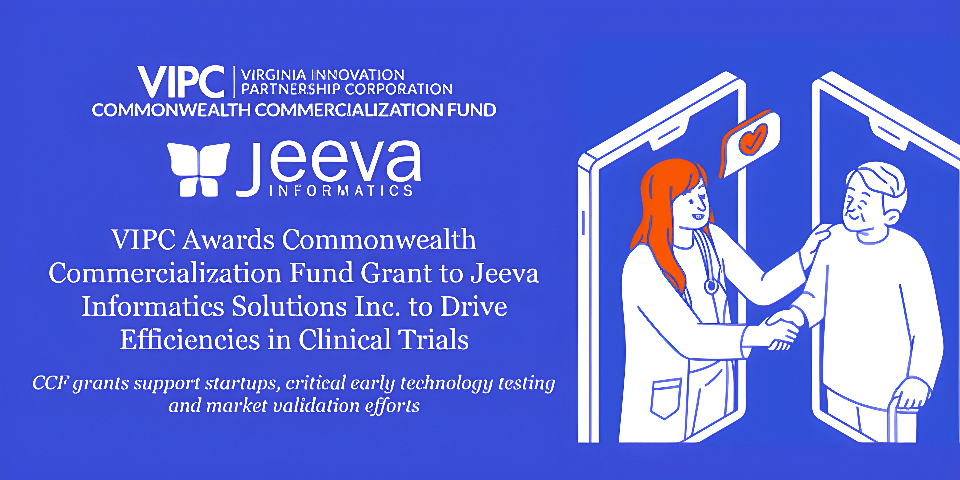
Source: EIN News CCF grants support startups, critical early technology testing and market validation efforts. RICHMOND, VIRGINIA, UNITED STATES, June 27, 2023/EINPresswire.com/ — The Virginia

Source: Cision PRweb The FDA has released new guidance for decentralized clinical trials (DCTs) for drugs, biological products, and medical devices to improve DCT implementation.

Source: PR Distribution Genomics expert and social entrepreneur, Harsha Rajasimha, looks to bridge OMICS and Exposure Health with partner DailyBreath at the inaugural total exposure

Source: Cision PRweb The value of life-saving drugs for patients suffering from serious diseases is priceless. But the current economic environment is stifling the biopharmaceutical
Jeeva Clinical Trials Inc.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
Dr. Sweta Sneha is the Founder and Executive Director of Healthcare Management & Informatics and Professor of Information Systems & Security at Kennesaw State University. A globally recognized expert with a passion towards equity and diversity, Sneha is a strategic leader with two decades of experience in technology and informatics. She is a recipient of Distinguished Service Award, KSU Collaboration Award, NCWIT RISE-IT Award, and WIT Honoree, WIT Finalist (2018, 2019, 2020, 2021, 2022). Dr. Sneha has published over 100 peer-reviewed research; authored a book on revolutionizing healthcare; delivered panel sessions, workshops, and keynotes; in addition to serving on several boards. Dr. Sneha serves as editorial steward and university ambassador for Blockchain in Healthcare Today; editorial board for Health Systems Journal; co-chairs Health IT at AMCIS, and HICSS. Her leadership has led to enhancing KSU’s footprint on diversity and equity, STEAM education, and outreach. She has a PhD in Computer Information Systems from Georgia State University and a BS in Computer Science from University of Maryland. Prior to academia, she worked at PricewaterhouseCoopers in Management Consulting.