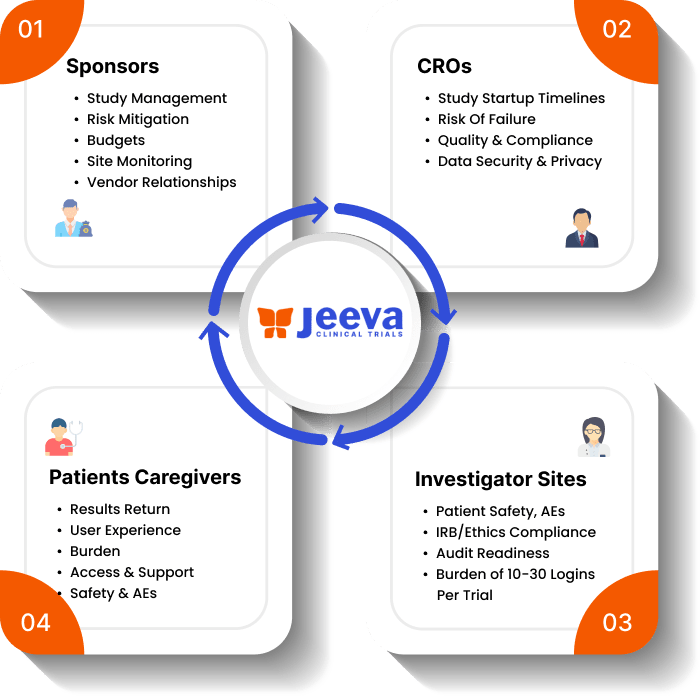
All while boosting engagement between researchers and patients by email, SMS, audio calling, and video calling with a single login for a study from any browser-enabled mobile device.
- Remotely screen, recruit and retain more participants faster
- Elevate electronic patient-reported outcomes (ePRO) to a whole new level during a live video call (vPRO).
- Directly capture electronic study data, lab reports, safety, medical history, and clinical outcomes assessments (eCOA) using the drag-and-drop form builder including during a live video call between investigator and patient.
- Minimize investigator burden by capturing patient self-reported information at a time and place convenient for the patient.
- Maximize patient satisfaction, by providing flexibility for them to complete study participation and follow-up visits remotely or in person.
- Reduce operational costs by minimizing the need for in-person monitoring visits as all study staff will have real-time access to the central clinical database.