We Supercharge All Aspects of Clinical Trial Management Under One-Login
Modernizing Clinical Research With Our Human-Centric Software & CRO Solutions
Are you the head of Clinical Operations at a Biopharmaceutical or medical device sponsor planning your next clinical trial within the next six months?
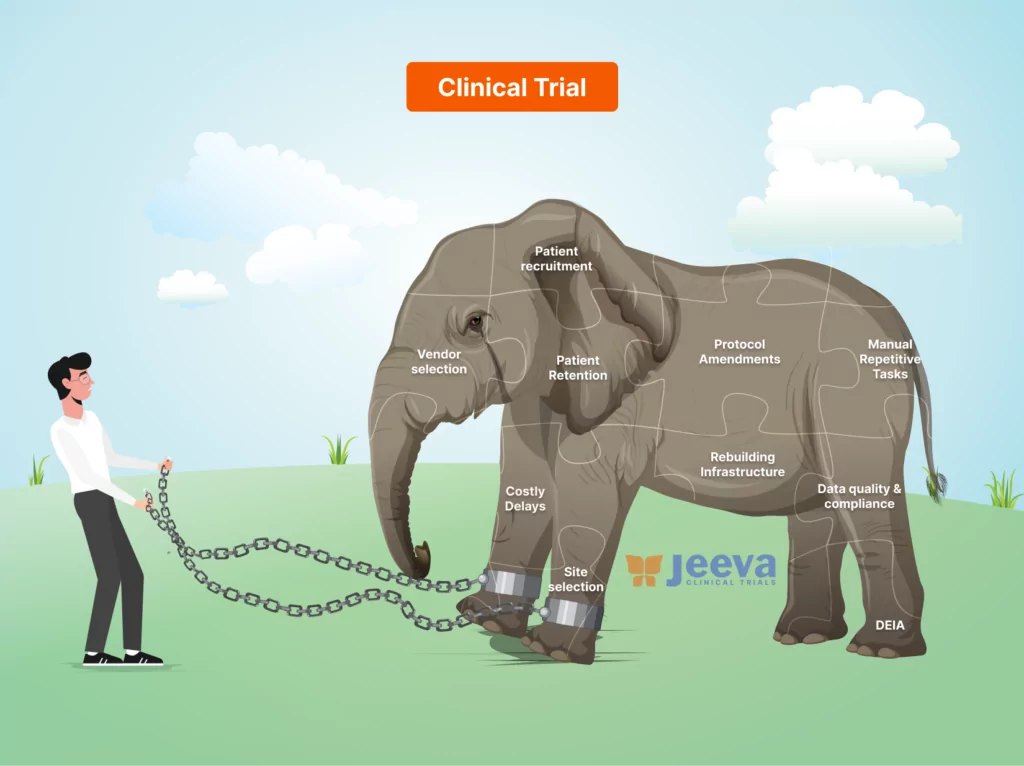
Is speed, efficiency, vendor selection, management, quality, regulatory compliance, or cost at the top of mind? A Clinical Trial is a collaborative effort among many functional leaders relying on data, technology, people, and standard operating procedures for execution. Ultimately, it’s you, the study manager, who has to bring it all together to make a study successful.
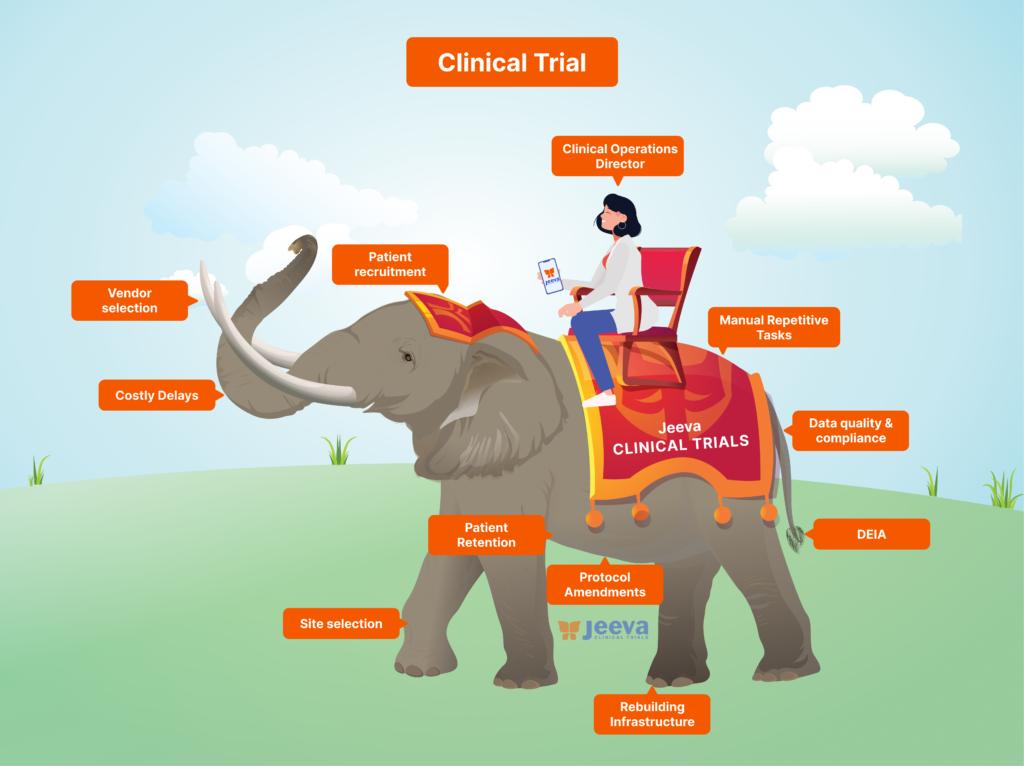
Team Jeeva listened to over 5000+ stakeholders of clinical operations at sponsors, investigator sites, participants, caregivers, CROs, and regulators to understand their perspectives on the severe inefficiencies in the industry. We built a unified solution that puts you in the driver’s seat.