
Highlights of Virginia Small Business Success Stories
WASHINGTON, D.C. May 2, 2022 Today, U.S. Senator Tim Kaine is highlighting small business success stories from across the Commonwealth in honor of National Small
Jeeva Trials > News

WASHINGTON, D.C. May 2, 2022 Today, U.S. Senator Tim Kaine is highlighting small business success stories from across the Commonwealth in honor of National Small

As new non-COVID-related clinical trials proliferate, the need for effective global decentralized CT management is becoming even more apparent. What is needed, says Dr. Harsha

Source: MedCity News Conducting a successful clinical trial depends on well-designed patient recruitment procedures and retaining the research participants until the completion of the study.

JoTo PR Disruptors’ signs Jeeva Trials, a human-centric Software as a Service (SaaS) company that is working to revolutionize the clinical trials space at scale.

Clinical trials are expensive, take significant time, and can run into any number of challenges and delays. The average drug study will see a 30%
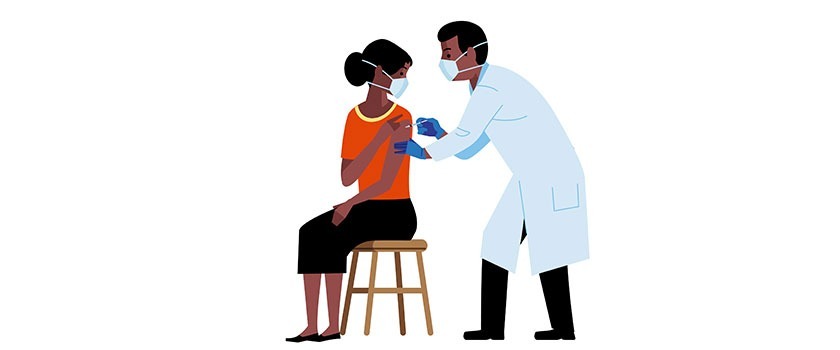
Source: PubsOnline Over the past two years, the testing of new medical devices, drugs and vaccines has increasingly been performed outside large medical centers. Faster

Source: ACRP There’s more to the quest of fostering diversity, equity, and inclusion in the delivery of clinical research opportunities than just reaching race/ethnicity-, gender-,

Maximizing Clinical Trial Access, Diversity, and Efficiency are Keys to Optimizing Orphan Drug Development February 22, 2022 February is Rare Disease Month—an important time when

Source: Insightcare By Harsha Rajasimha, PhD, Founder and CEO, Jeeva Clinical Trials Inc. www.jeevatrials.com To say that COVID-19 has been a challenge to healthcare systems

Source: MarketScale Clinical trials can save lives and provide new ways to treat disease. The industry estimates that 85% of all clinical trials face delays

The loss of his daughter to a rare congenital disorder in the United States and the passing of his younger brother in India from complications

The growing use of decentralized clinical trials has raised serious concerns regarding verification of patient comprehension and proper informed consent. The solution, says Dr. Harsha
Source: Healthcare Weekly Almost everything went online due to the COVID-19 pandemic, from remote collaboration with coworkers to ordering groceries and conducting online classes. But

Source: Clinical Trials Arena Diversity is a growing concern in all aspects of modern life, but the ramifications of this disparity in health care can

MANASSAS, VA. (PRWEB) DECEMBER 01, 2021 The ongoing COVID-19 pandemic ranks as perhaps the most serious global health crisis of the past hundred years. Worldwide,

Source: Relias Media IRBs are seeing a surge in study protocols that include remote recruitment, consent, and participation, raising ethical and regulatory considerations for investigators.

Source: HR.com Serious inefficiencies are hampering clinical trials of new drugs and treatments, prolonging the suffering of millions. Today’s platform technologies, says Dr. Harsha Rajasimha

Source: Pharmaceutical Outsourcing The outbreak of the COVID-19 pandemic seriously disrupted new drug testing, treatments, and in-person healthcare services; access to clinical trial sites was

Source: Outsourcing-Pharma Leaders from the trial tech provider discuss how human-focused technology on eClinical platforms can tackle inefficiencies associated with traditional studies. According to Harsha

Serious inefficiencies are hampering clinical trials of new drugs and treatments, prolonging the suffering of millions. Today’s platform technologies, says Dr. Harsha Rajasimha of Jeeva
Jeeva Clinical Trials Inc.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
Dr. Sweta Sneha is the Founder and Executive Director of Healthcare Management & Informatics and Professor of Information Systems & Security at Kennesaw State University. A globally recognized expert with a passion towards equity and diversity, Sneha is a strategic leader with two decades of experience in technology and informatics. She is a recipient of Distinguished Service Award, KSU Collaboration Award, NCWIT RISE-IT Award, and WIT Honoree, WIT Finalist (2018, 2019, 2020, 2021, 2022). Dr. Sneha has published over 100 peer-reviewed research; authored a book on revolutionizing healthcare; delivered panel sessions, workshops, and keynotes; in addition to serving on several boards. Dr. Sneha serves as editorial steward and university ambassador for Blockchain in Healthcare Today; editorial board for Health Systems Journal; co-chairs Health IT at AMCIS, and HICSS. Her leadership has led to enhancing KSU’s footprint on diversity and equity, STEAM education, and outreach. She has a PhD in Computer Information Systems from Georgia State University and a BS in Computer Science from University of Maryland. Prior to academia, she worked at PricewaterhouseCoopers in Management Consulting.