eConsent as a Service Highlights
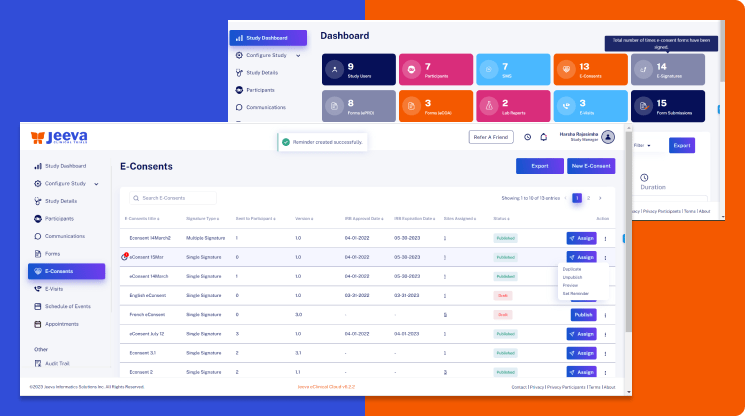
Jeeva remote eCaaS is an innovative SaaS-based collaboration platform hosted on the secure AWS cloud designed to assist clinical researchers. It facilitates the remote screening and recruitment of participants while ensuring full compliance with regulations by collecting accurate informed consent.
The eCaaS solution enables social media outreach to targeted groups, minimizes costly screen failures, and boosts success rates. It helps avoid information overload for patients and builds trust by organizing all trial information in one accessible central location, accelerating the recruitment process and timelines.
The eCaaS solution enables social media outreach to targeted groups, minimizes costly screen failures, and boosts success rates. It helps avoid information overload for patients and builds trust by organizing all trial information in one accessible central location, accelerating the recruitment process and timelines.