Our Clinical Trials Visits Scheduler Highlights
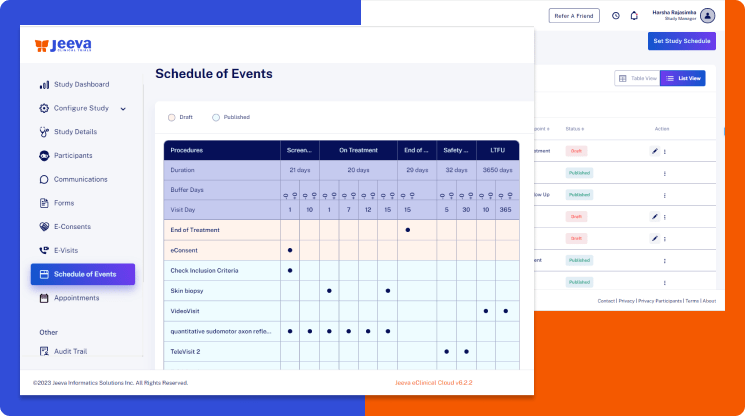
The Jeeva visits scheduling platform makes it easier to configure the patient visit schedule in a centralized location.
So, when the time comes to implement protocol amendments, the Jeeva centralized schedule of visits table maps all procedures to their respective ePRO, eCOA, eCRF, eConsent, eVisit, and other lab procedures.
This mapping enables the visits scheduler system to automate validations, protocol deviations, reminders, notifications, form assignments, appointment scheduling, and other user workflows to bring unprecedented efficiencies to trial management.
Every clinical trial protocol has a unique schedule of visits table of one or more procedures one should complete during each clinical study visit.
So, when the time comes to implement protocol amendments, the Jeeva centralized schedule of visits table maps all procedures to their respective ePRO, eCOA, eCRF, eConsent, eVisit, and other lab procedures.
This mapping enables the visits scheduler system to automate validations, protocol deviations, reminders, notifications, form assignments, appointment scheduling, and other user workflows to bring unprecedented efficiencies to trial management.
Every clinical trial protocol has a unique schedule of visits table of one or more procedures one should complete during each clinical study visit.