Revolutionizing Interventional Radiology: How Jeeva and IR Centers USA are Transforming Patient Outcomes
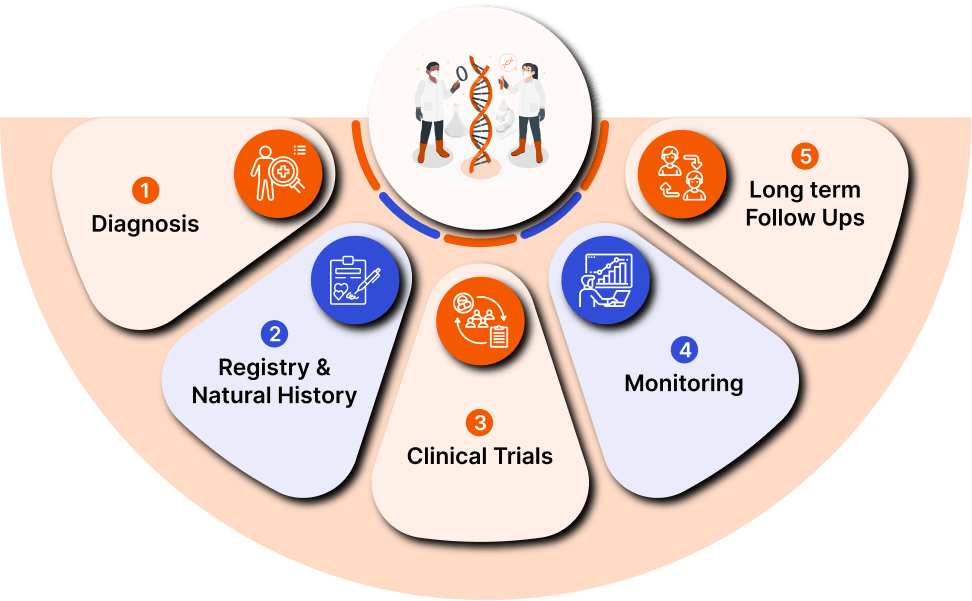
IR Centers™️ leads Interventional Radiology, offering cutting-edge, minimally invasive treatments in Urology, Gastroenterology, and Orthopedics. With top physician leaders, advanced technology, and nationwide partnerships, they prioritize patient-centric care for exceptional outcomes. As a network partner of Prostate Centers USA®, Hemorrhoid Centers®, and Ortho Centers™️, they are committed to innovation and collaboration in healthcare.