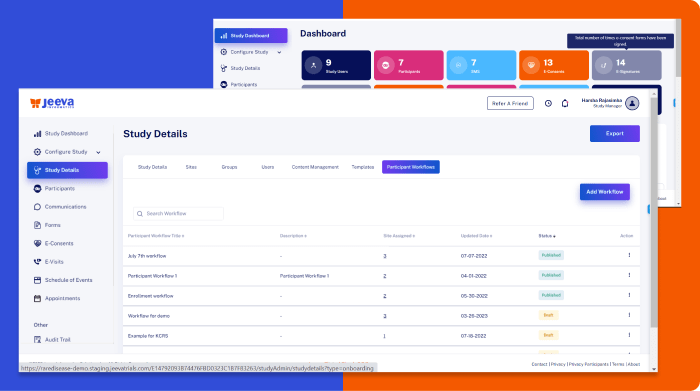
Electronic Data Capture Integrated with Randomization and Clinical Trial Management System
We designed powerful user interface design and experiences to streamline clinical data capture with standard vocabularies such as MedDRA and enabling real-time collaboration, reducing human error and maintaining the integrity of the research data.
Jeeva EDC software effectively manages large data volumes, enhancing the speed and accuracy of data processing while reducing the administrative load on clinical staff.
Jeeva’s EDC system fully complies with GCP (Good clinical practice), FDA (Food and drug administration), EMA (European medicines agency), and ICH (International code of harmonization) guidelines, tracking and recording every data point to generate detailed audit trails that ensure complete transparency and compliance throughout the trial process.
Jeeva EDC software effectively manages large data volumes, enhancing the speed and accuracy of data processing while reducing the administrative load on clinical staff.
Jeeva’s EDC system fully complies with GCP (Good clinical practice), FDA (Food and drug administration), EMA (European medicines agency), and ICH (International code of harmonization) guidelines, tracking and recording every data point to generate detailed audit trails that ensure complete transparency and compliance throughout the trial process.