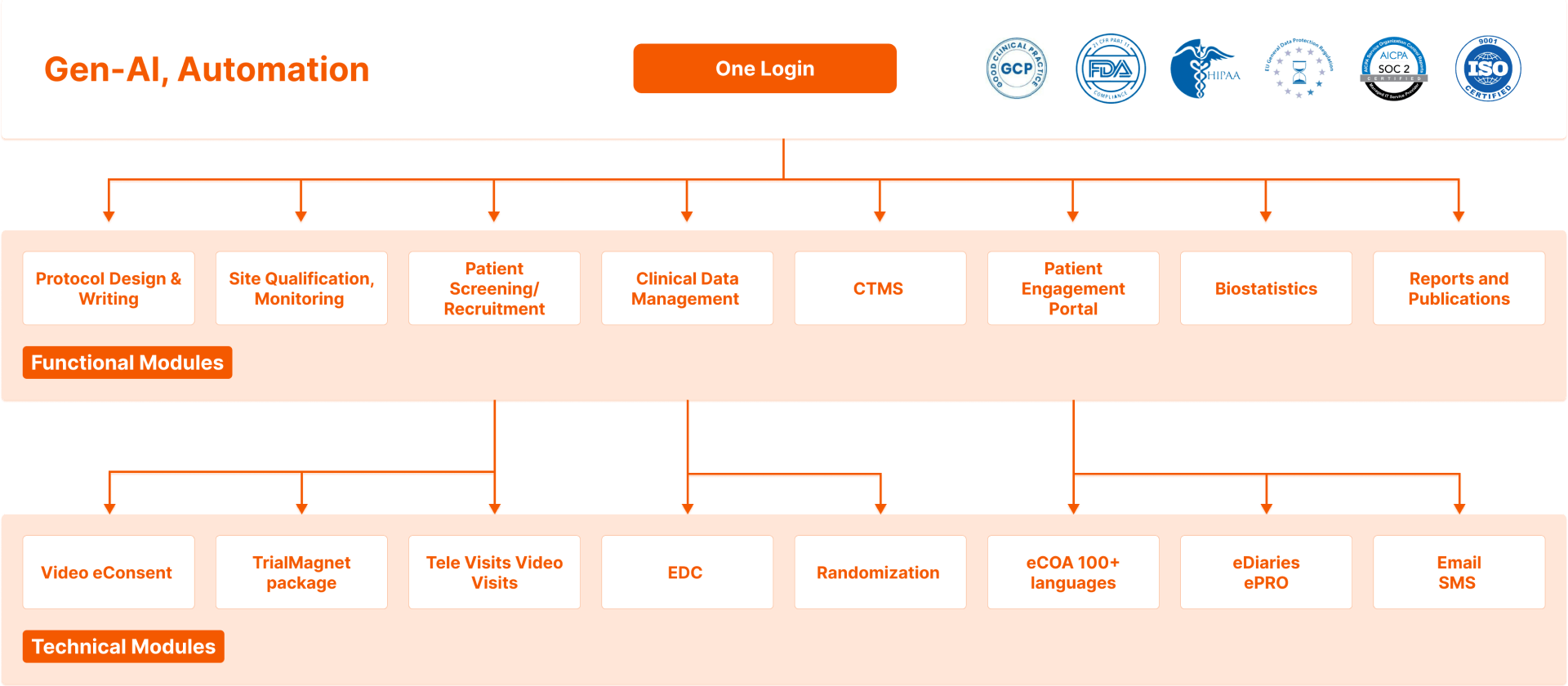
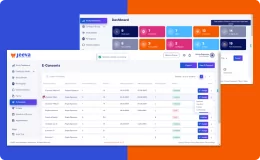
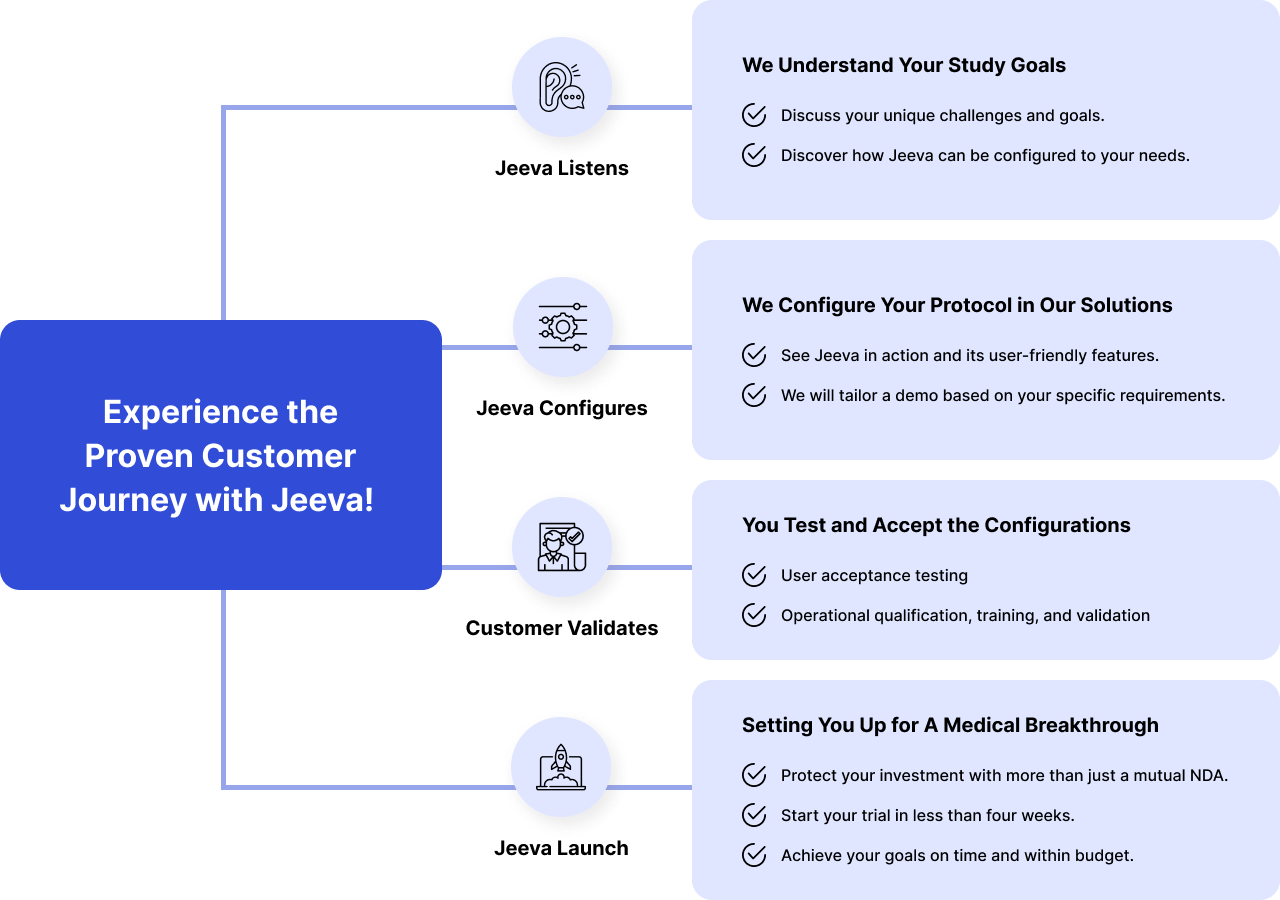
- Eliminate data errors with automated edit checks, branching logic, and scoring.
- Improved Security with our SOC2 and HIPAA-compliant data protection.
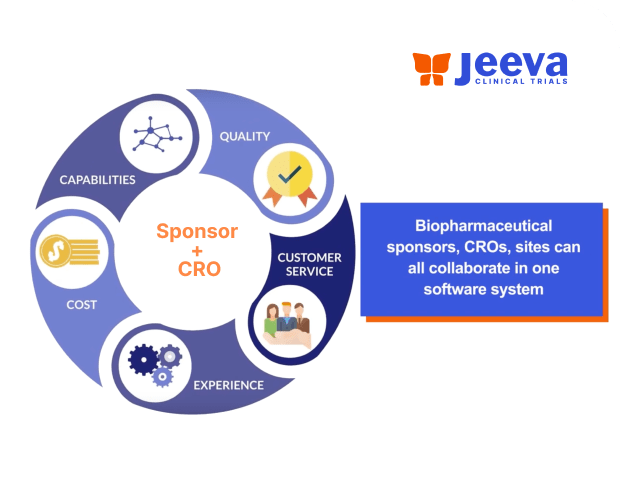
- Reduce Costs by eliminating the need for various point solutions.
- Quicker Time to First Patient First Visit.
- Keep participants engaged in 100+ languages.
- 3X your time to Completion.
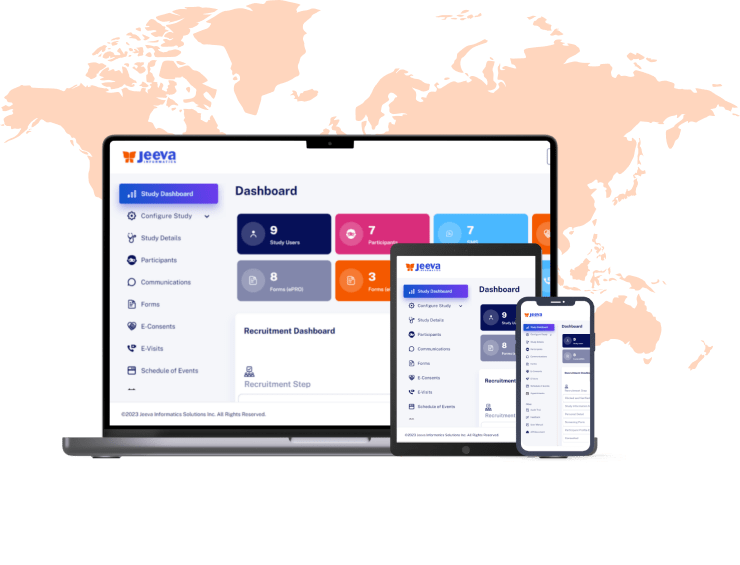
- On-the-go data collection and engagement from any device.
- Intuitive Interface with effortless navigation
- Premium 24/7 customer support!