Jeeva eClinical Platform Highlights
Our eClinical system empowers sponsors, CROs, and Investigator teams to Implement modern Clinical Trials by boosting the efficiency of operations and maximizing universal patient access.
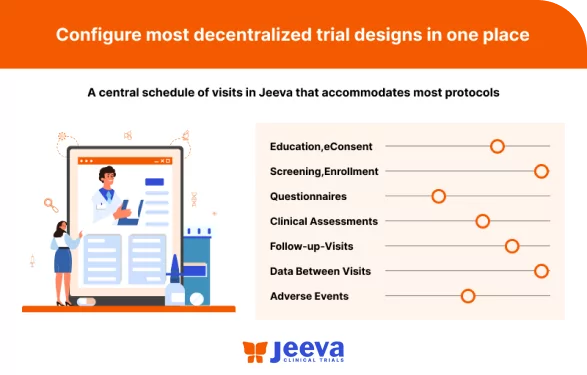
The decentralized clinical trials solution allows sponsors to execute efficient trials in single or multiple centers to improve accessibility, engage remote participants from the convenience of their homes, and eliminate the need for multiple-point solutions with one unified platform. We also help configure versatile trial protocol designs and minimize manual effort with the intelligent automation built into the platform.
The decentralized clinical trials solution allows sponsors to execute efficient trials in single or multiple centers to improve accessibility, engage remote participants from the convenience of their homes, and eliminate the need for multiple-point solutions with one unified platform. We also help configure versatile trial protocol designs and minimize manual effort with the intelligent automation built into the platform.