Customer: Cure Rare Disease
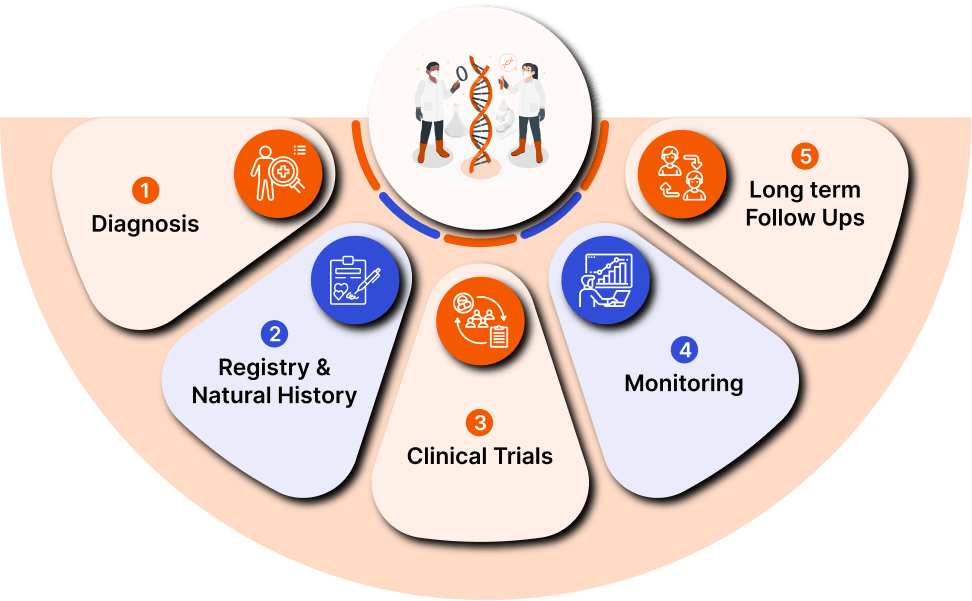
Founded by a rare dad on a mission to make orphan drug clinical trials universally accessible, Jeeva’s software solutions are designed to empower rare disease research by engaging patients through the clinical trial journey.